lv reversible vs unreversible | reversible vs irreversible volume lv reversible vs unreversible We distinguish between two kinds of irreversible processes. A process that . 551. 47K views 5 years ago. Fear the Walking Dead Survival is a unique, immersive thrill experience. Check out this walk-through of the attraction in the heart of downtown Las Vegas at.
0 · reversible vs irreversible work
1 · reversible vs irreversible volume
2 · reversible vs irreversible process
3 · reversible vs irreversible pressure
4 · reversible vs irreversible gas
5 · reversible vs irreversible
6 · irreversible process vs reversible state
7 · example of reversible and irreversible process
Favourite Bag. £2,200.00. Item Unavailable. Discover our latest designer Monogram Empreinte Leather, Women collection exclusively on louisvuitton.com and in Louis Vuitton Stores.
reversible vs irreversible work
A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference. From our definitions of reversible and irreversible pressure–volume work, we .
reversible vs irreversible volume
A reversible process is one in which both the system and its environment can return to exactly the states they were in by following the .
We distinguish between two kinds of irreversible processes. A process that .
We distinguish between two kinds of irreversible processes. A process that .
A reversible process implies there is no entropy generated as the system moves between two .
For reversible processes (the most efficient processes possible), the net change in entropy in .A reversible process is truly an ideal process that rarely happens. We can make certain .
In animal studies, rapid atrial pacing has been shown to decrease the left ventricular ejection .
reversible vs irreversible process
A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference. From our definitions of reversible and irreversible pressure–volume work, we have \({dw}^{rev}<{dw}^{irrev}\) and\(\ w^{rev}
We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process.A reversible process implies there is no entropy generated as the system moves between two states (Sgen = 0 S g e n = 0). This does not mean that the entropy is constant. When the entropy remains constant (ΔS = 0 Δ S = 0), and the process is called isentropic.For reversible processes (the most efficient processes possible), the net change in entropy in the universe (system + surroundings) is zero. Phenomena that introduce irreversibility and inefficiency are: friction, heat transfer across finite temperature differences, free expansion, .
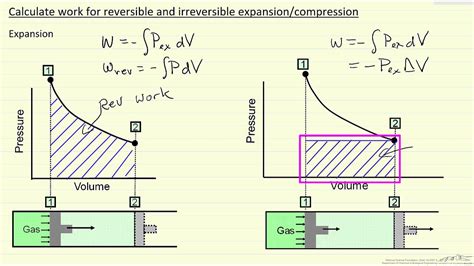
The diagram looks exactly how it should for a reversible process \to 1$. If the process is irreversible, on the other hand, the smooth solid line \to 1$ is deceptive, for it suggests that the system is passing through a sequence of equilibrium states in the process \to 1$. This is not the correct way to represent an irreversible process.
A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference.
In animal studies, rapid atrial pacing has been shown to decrease the left ventricular ejection fraction (LVEF) by roughly 52%. 3 Similarly, a 36% drop in cardiac index and 34% increase in cardiac size have been shown to occur with rapid right ventricular pacing. 4.A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference. From our definitions of reversible and irreversible pressure–volume work, we have \({dw}^{rev}<{dw}^{irrev}\) and\(\ w^{rev}
We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process. We distinguish between two kinds of irreversible processes. A process that cannot occur under a given set of conditions is said to be an impossible process. A process that can occur, but does not do so reversibly, is called a possible process or a spontaneous process.
A reversible process implies there is no entropy generated as the system moves between two states (Sgen = 0 S g e n = 0). This does not mean that the entropy is constant. When the entropy remains constant (ΔS = 0 Δ S = 0), and the process is called isentropic.For reversible processes (the most efficient processes possible), the net change in entropy in the universe (system + surroundings) is zero. Phenomena that introduce irreversibility and inefficiency are: friction, heat transfer across finite temperature differences, free expansion, . The diagram looks exactly how it should for a reversible process \to 1$. If the process is irreversible, on the other hand, the smooth solid line \to 1$ is deceptive, for it suggests that the system is passing through a sequence of equilibrium states in the process \to 1$. This is not the correct way to represent an irreversible process.A reversible process is truly an ideal process that rarely happens. We can make certain processes close to reversible and therefore use the consequences of the corresponding reversible processes as a starting point or reference.
did hermes invent sandals

reversible vs irreversible pressure
reversible vs irreversible gas
reversible vs irreversible
Spēlējiet tiešsaistes kazino spēles Fenikss Casino populārākajā online kazino Latvijā 200 Bezriska Griezieni un € 300 kazino bonuss ️ Pievienojies TAGAD!
lv reversible vs unreversible|reversible vs irreversible volume